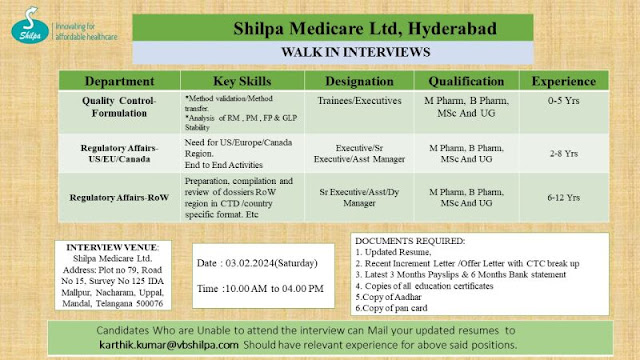
Shilpa Medicare Ltd Walk In Interview For Quality Control and Regulatory Affair Dept
1) Department: Quality Control- Formulation
Key Skills:
Method validation Method transfer
Analysis of RM, PM, FP & GLP Stability
Designation: Trainees/Executives
Qualification: M Pharm, B Pharm, MSc And UG
Experience: 0-5 Yrs
2) Department: Regulatory Affairs- US/EU/Canada
Key Skills:
Need for US/Europe/Canada Region. End to End Activities
Designation: Executive/ Sr Executive/ Asst Manager
Qualification: M Pharm. B Pharm. MSc And UG
Experience: 2-8 Yrs
3) Department: Regulatory Affairs-RoW
Key Skills: Preparation, compilation and review of dossiers RoW region in CTD/country specific format. Etc
Designation: Sr Executive/Asst/Dy Manager
Qualification: M Pharm, B Pharm. MSc And UG
Experience: 6-12 Yrs
Shilpa Medicare Walk In Interview Details:
Date: 03.02.2024(Saturday)
Time:10.00 AM to 04.00 PM
Venue:
Shilpa Medicare Ltd.
Address: Plot no 79, Road No 15. Survey No 125 IDA Mallpur, Nacharam, Uppal, Mandal, Telangana 500076
Below Document Required:
1. Updated Resume,
2. Recent Increment Letter /Offer Letter with CTC break up 3. Latest 3 Months Payslips & 6 Months Bank statement
4. Copies of all education certificates
5.Copy of Aadhar
6.Copy of pan card
 |
Shilpa Medicare Ltd Walk In Interview For Quality Control and Regulatory Affair Dept |
Post a Comment