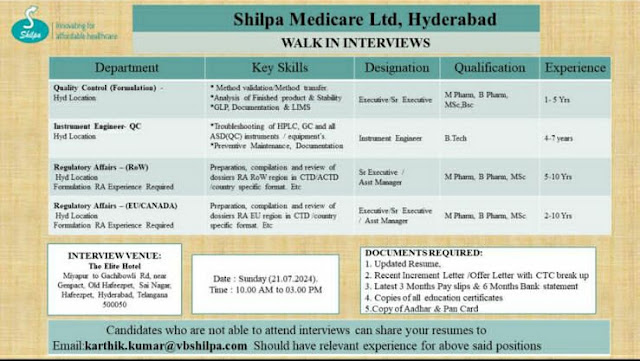
Shilpa Medicare Walk In Interview For QC/ Regulatory Affairs/ Instrumentation
1) Department : Quality Control (Formulation)
Hyd Location
Key Skills :
• Method validation/Method transfer
• Analysis of Finished product & Stability
• GLP, Documentation & LIMS
Designation : Executive Sr Fixecutive
Qualification : M Pharm, B Pharm MSc Bio
Experience : 1-5 Yrs
2) Department : Instrument Engineer QC
Hvd Location
Key Skills :
• Troubleshooting of HPLC, GC and all ASD(QC) instruments/equipment's
• Preventive Maintenance, Documentation
Designation : Instrument Engineer
Qualification : B.Tech
Experience : 4-7 Yrs
3) Department : Regulatory Affairs -(RoW)
Hvd Location
Formation RA Experience Required
Key Skills : Preparation, complation and review of dossiers RA Row region in CID ACTD country specific format. Etc
Designation : Se Executive Asit Manager
Qualification : M Pharm, B Pharm, MSc
Experience : 5-10 Yrs
4) Department : Regulatory Affairs (EU/CANADA)
Hyd Location
Formation RA Experience Required
Key Skills : Preparation, compilation and review of dossiers RA EU region in CTD /country specific format. Etc
Designation : Executive St Executive Asst Manager
Qualification : M Pharm, B Pharm, MSc
Experience : 2-10 Yrs
Walk In Interview Details
Date: Sunday (21.07.2024).
Time: 10.00 AM to 03.00 PM
Venue: The Elite Hotel
Mirapur to Gachbowli Rd, nem Gempact, Old Hafeezpet, Sai Nagar Hafeezpet. Hyderabad, Telangana 500050
Documents Required :
1. Updated Resume.
2. Recent Increment Letter /Offer Letter with CTC break up 3. Latest 3 Months Pay slips & 6 Months Bank statement
4. Copies of all education certificates
5. Copy of Aadhar & Pan Card
 |
| Shilpa Medicare Walk In Interview For QC/ Regulatory Affairs/ Instrumentation |
إرسال تعليق